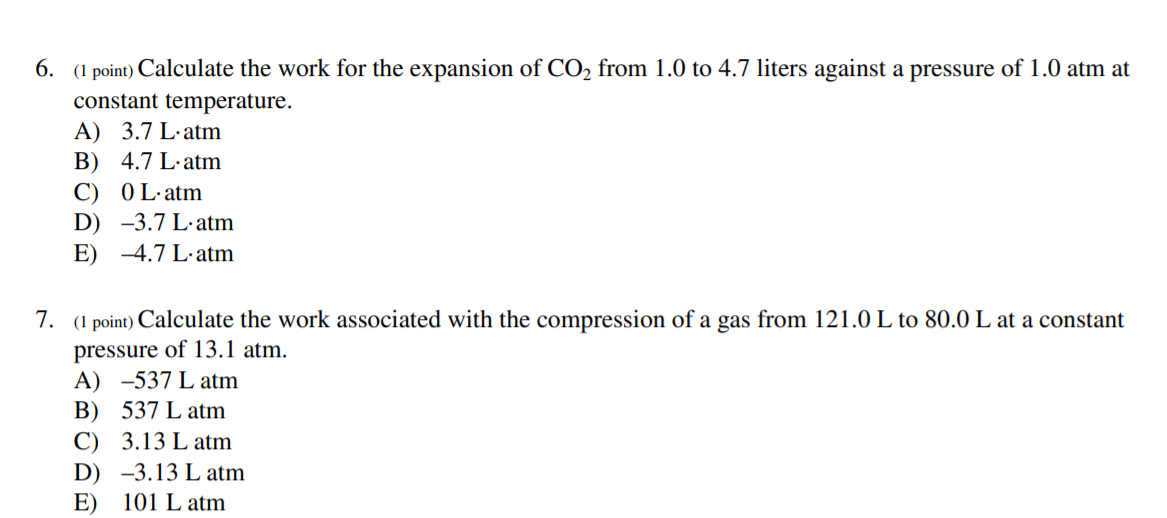
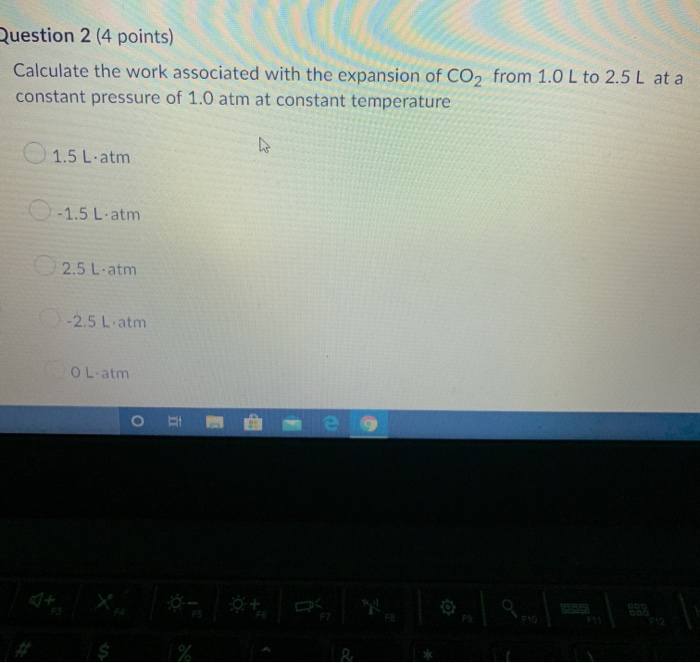
Chapter 2 Chem.docx - Ans: d Calculate the work for the expansion of CO2 from 1.0 to 5.8 liters against a pressure of 1.0 atm at constant | Course Hero

Calculate maximum work At 303k ,22×10^-³ kg of co2 was expanded isothermally and reversibly from 11 2dm^3 to 16 8 - Chemistry - - 13575559 | Meritnation.com

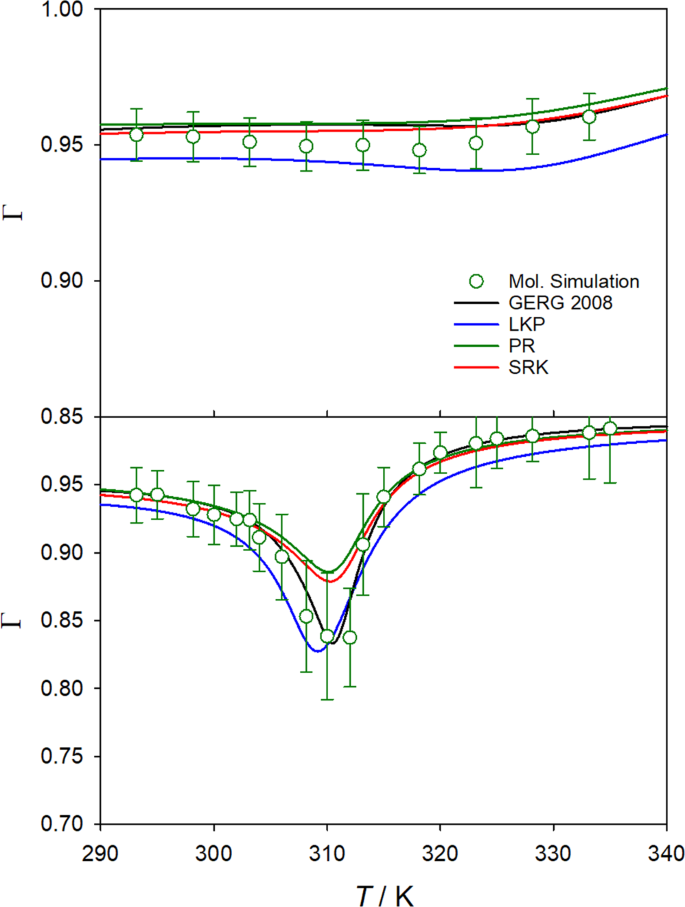
Depressurization of CO2 in a pipe: High-resolution pressure and temperature data and comparison with model predictions - ScienceDirect

Solubility of Carbon Dioxide in Aqueous Solutions of Monoethanolamine in the Low and High Gas Loading Regions | Journal of Chemical & Engineering Data

SOLVED: (10 pts) Calculate the maximum non-expansion work per mole that may be obtained from fuel cell in which the chemical reaction is the combustion of methane at 298 K. CHa(g) Oz(g)
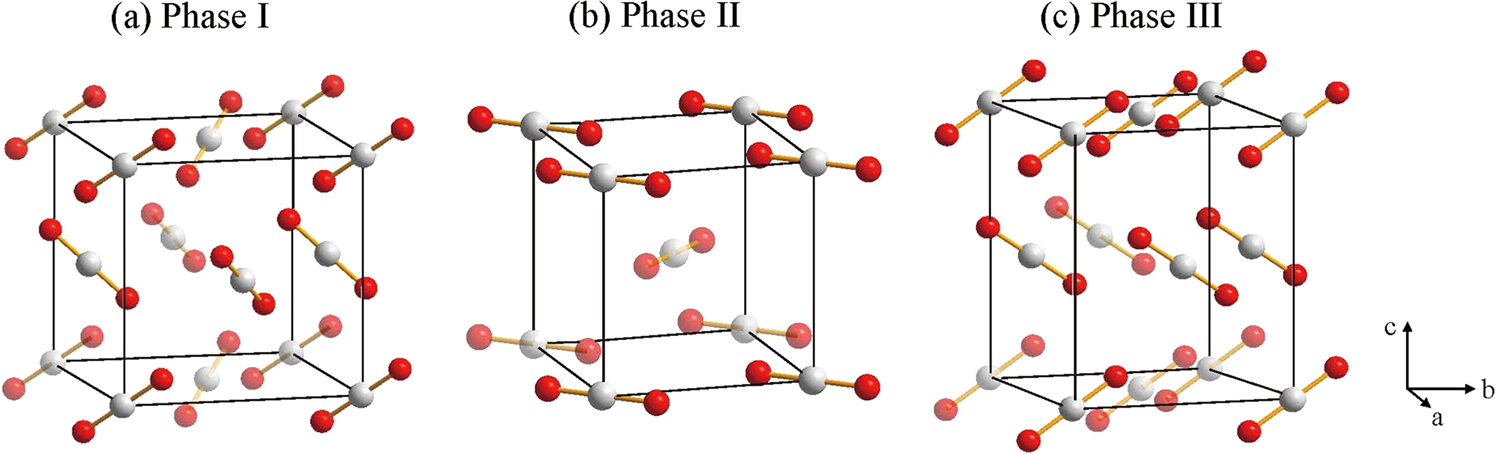
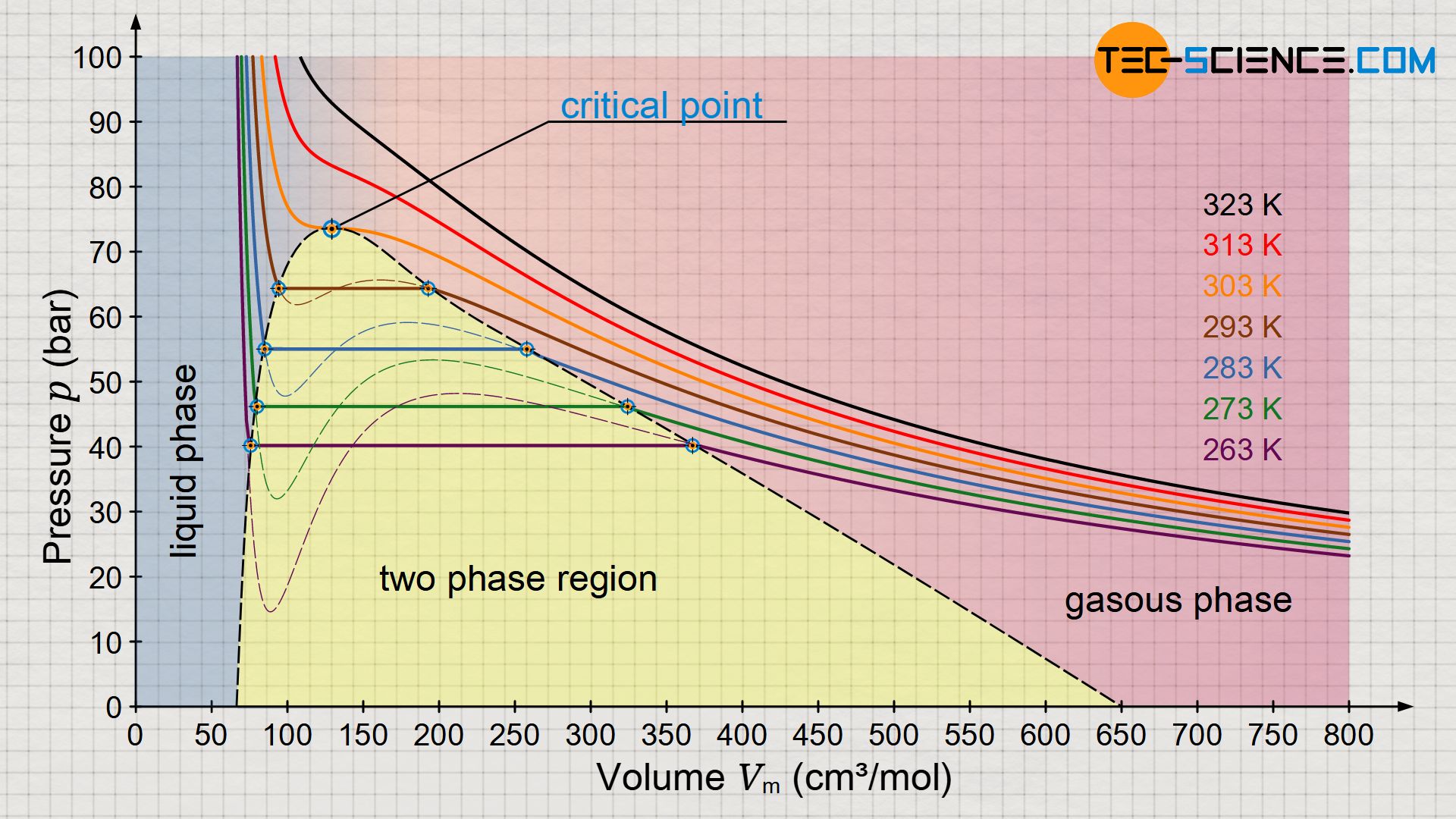
Predicting the phase diagram of solid carbon dioxide at high pressure from first principles | npj Quantum Materials
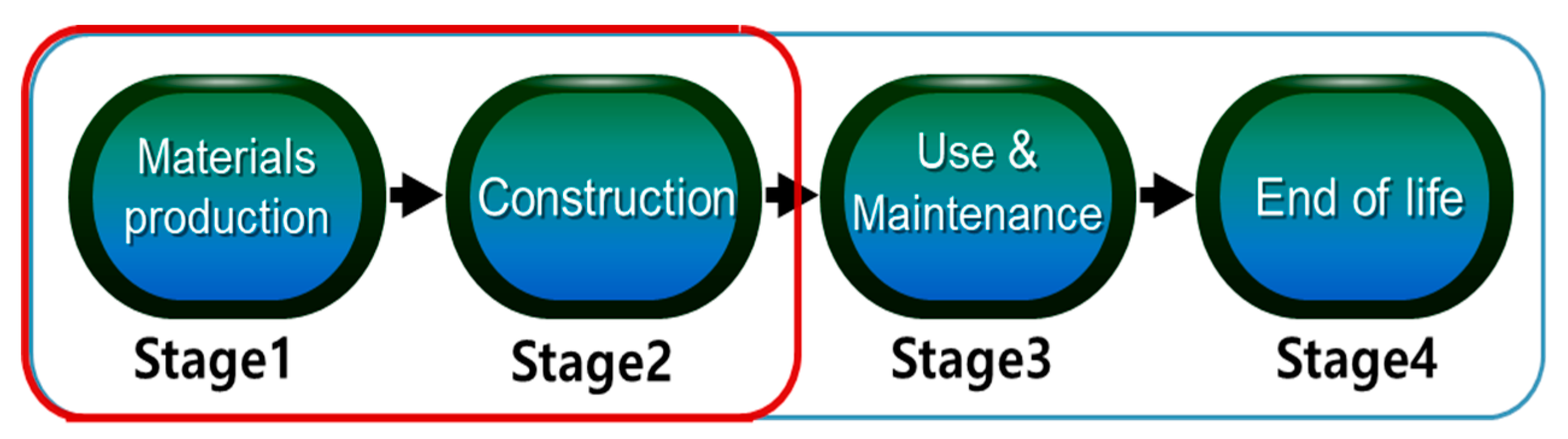
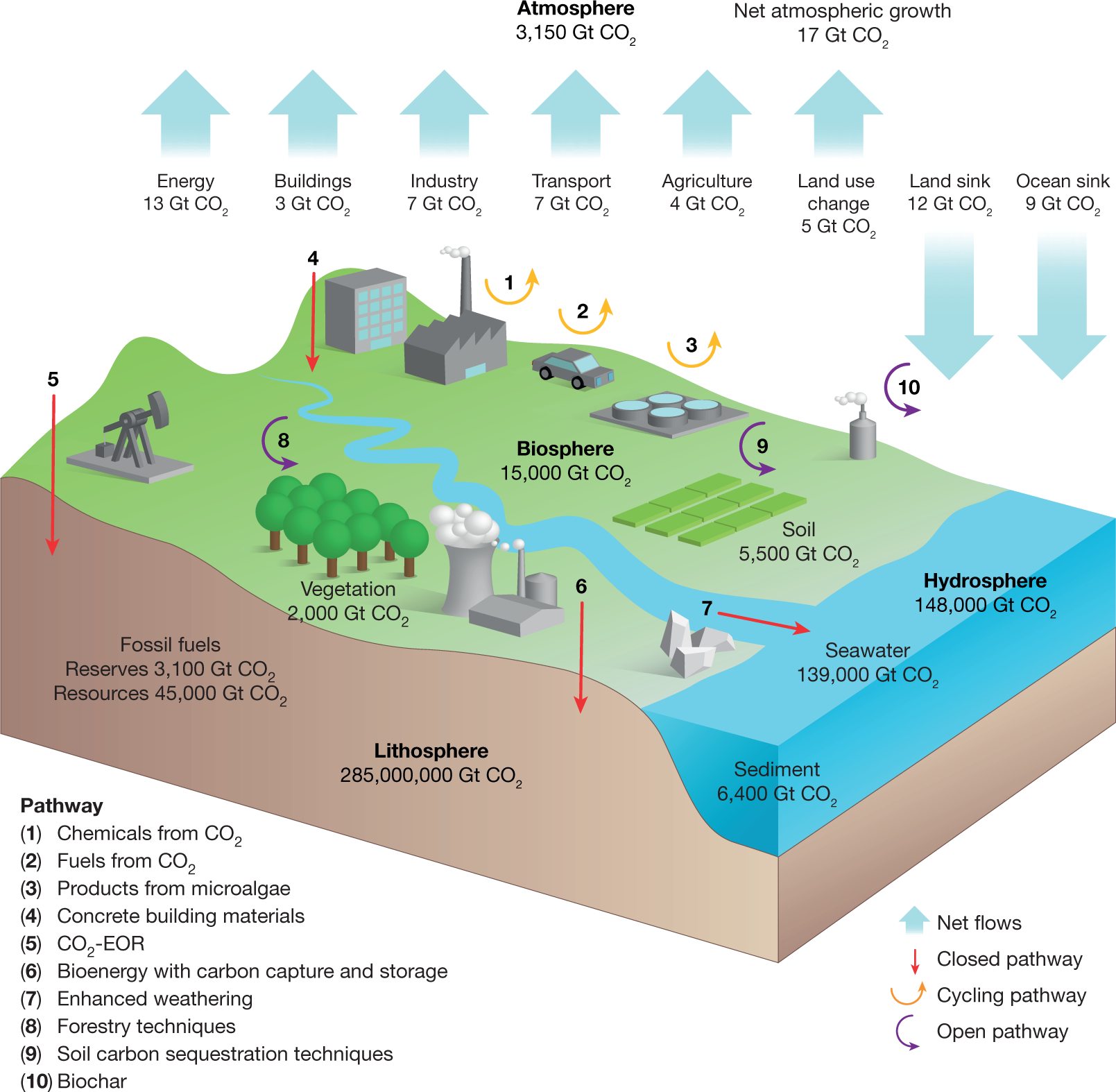
Applied Sciences | Free Full-Text | CO2 Emission Calculation Method during Construction Process for Developing BIM-Based Performance Evaluation System

SOLVED: Problem Set#9 Thermochemistry Submitted You received no credit for this question in the previous att 2 Enter your answer in the provided box 393-g quantity of dry ice (solid carbon dioxide)

Experimental Modeling and Optimization of CO2 Absorption into Piperazine Solutions Using RSM-CCD Methodology | ACS Omega